Categories & Sectors: Alternating, Full Air Replacement Mattresses, Mattresses
Description
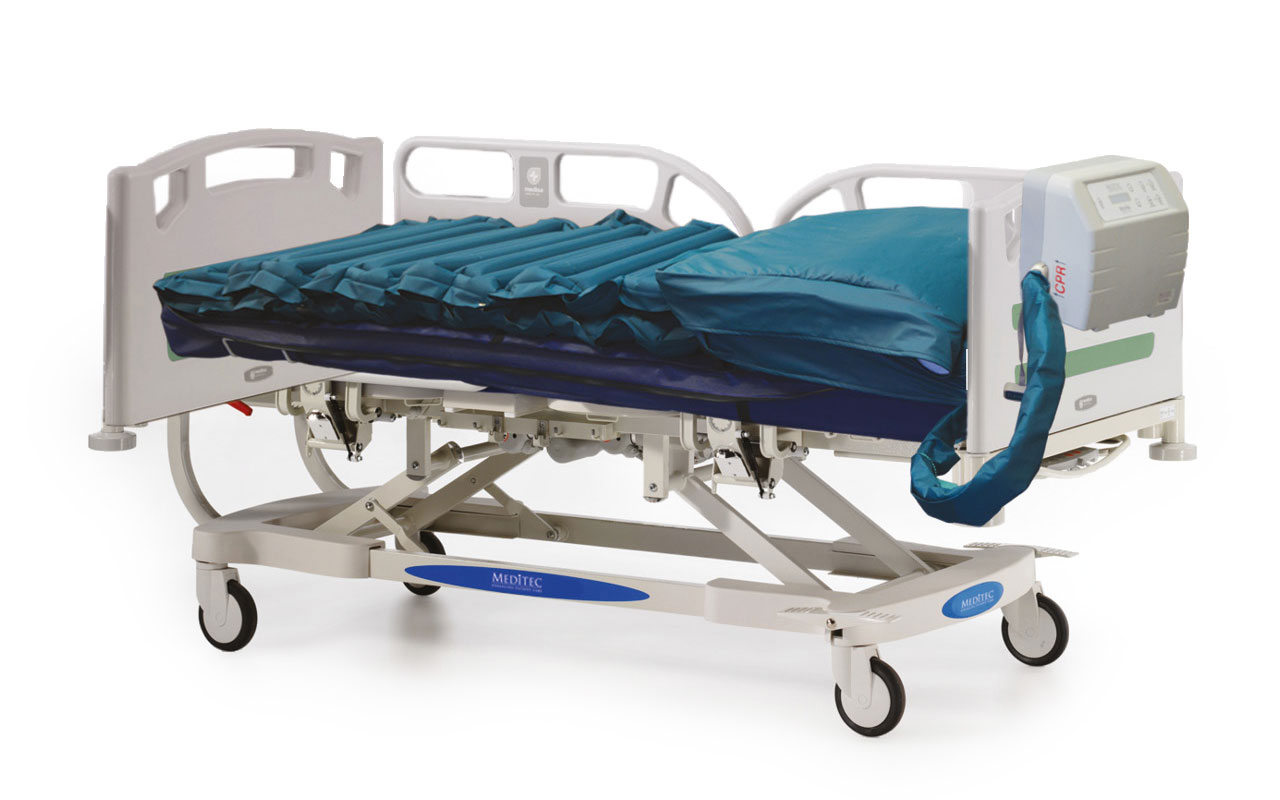
Advanced Alternating Therapy for Enhanced Pressure Relief.
The MediAir alternating air mattress is a clinically proven pressure relieving mattress suitable for patients at very high risk and caters for all grades of pressure ulcers.
Automatically stays inflated for 21 days in the event of a power-cut.
MediAir Features
- Enhanced pressure relief due to shorter cycle times of 5-20 minutes
- One touch max inflate
- Multi-stretch, waterproof polyurethane cover reduces the risk of shear and friction
- CPR mode allows for rapid deflation
- Simple and easy to use control unit
- Maximum user weight 180kg
- Automatically stays inflated for 21 days in the event of a power-cut
- Suitable for up to, and including, grade 4 pressure ulcer
- Inbuilt Tilt Sensor also detects when bed is profiled and ensures extra air is applied to protect from bottoming out
- Transport facility allowing unpowered support of the patient for up to 48 hours
- Custom sizes available
Technical Specification
| Air Mattress Specification | |
| Risk | Very High |
| Waterlow Scale | 20 |
| Max User Weight | 180kg |
| Warranty | 1 year |
| Length | 200cm |
| Width | 86cm |
| Height | 20cm/12cm (Fully inflated) |
| Cell Construction | Dual layered up to 28 upper cells |
| Air Supply Controller Specification | |
| Length | 33cm |
| Height | 24cm |
| Depth | 16cm |
| Weight | 1.5kg |
| Alternating Cycle Time | 5-20 mins |
| Alternating Cycle Type | 1 in 2 |
Based on standard size, contact us for any custom requirements.